
Expert Strategies: From Idea to Market Entry
Transforming innovative healthcare concepts into impactful, market-ready products is no small feat, especially in a field as demanding as healthcare. With a blend of medical expertise, strategic acumen, and hands-on execution, I have consistently delivered solutions that set new benchmarks. Here’s how I guide the process from concept to commercial success:
- Proven Innovations and Rapid Scaling:
I have developed world-first innovations like Video Laryngoscopes and a complete range of UVC disinfection devices, scaling these products from ₹0 to ₹30 crore in revenue within just 18 months. All these innovations are FDA 510(k) exempt, patented, and have successfully entered global markets, including the Middle East and the United States, while achieving profitability with no loans or liabilities—a rarity in the healthcare sector. - Rigorous Proof-of-Concept (PoC) Development:
Every product begins with a meticulous PoC phase, involving extensive testing in real-world scenarios. My UVC devices and laryngoscopes were validated in collaboration with leading institutions, ensuring they met the highest standards of effectiveness and safety. Through iterations and feedback, I transformed these early concepts into market-ready, first-in-the-world products. - Strategic Market Entry:
With a sharp focus on aligning products to both regulatory requirements and regional needs, I have enabled rapid market penetration. My products are now widely used in corporate hospitals, teaching colleges, and even food processing units, showcasing their versatility and value. - Localized Solutions for Global Adoption:
By adapting products to meet specific healthcare practices and regulations, I have ensured their relevance across diverse markets. For instance, these devices are 3D-printed for cost efficiency and precision, making them more accessible and practical for various applications. - Regulatory Expertise:
Navigating the complexities of global regulatory frameworks is a cornerstone of my approach. From domestic standards to FDA 510(k) exemptions, I ensure that compliance is seamless, minimizing delays and accelerating global market entry. - Broad Adoption and Academic Impact:
My devices have been adopted across major corporate hospitals, incorporated into research theses in teaching colleges, and have become a cornerstone of academic research. This adoption underscores their efficacy and credibility in diverse healthcare environments. - Collaborative Partnerships:
Through strategic alliances with academic and corporate institutions, I have ensured rigorous testing, validation, and scaling. These collaborations have solidified product credibility and expanded their reach, making them indispensable in their respective domains. - Financial Discipline and Strategic Growth:
My products achieved significant market impact without external funding, showcasing the power of financial independence and strategic planning. This approach has delivered sustainable growth, resilience, and exceptional valuations. - Media Recognition and Visibility:
By leveraging targeted media coverage and showcasing success stories, I ensure that each product gains the visibility needed to build trust, drive adoption, and maximize market impact.
From the conceptual stage to market leadership, I have consistently delivered healthcare innovations that meet unmet needs, set global standards, and drive measurable impact. Whether you are a start-up, investor, or institution, this expertise can help you turn visionary ideas into transformative realities.
Product Experience:
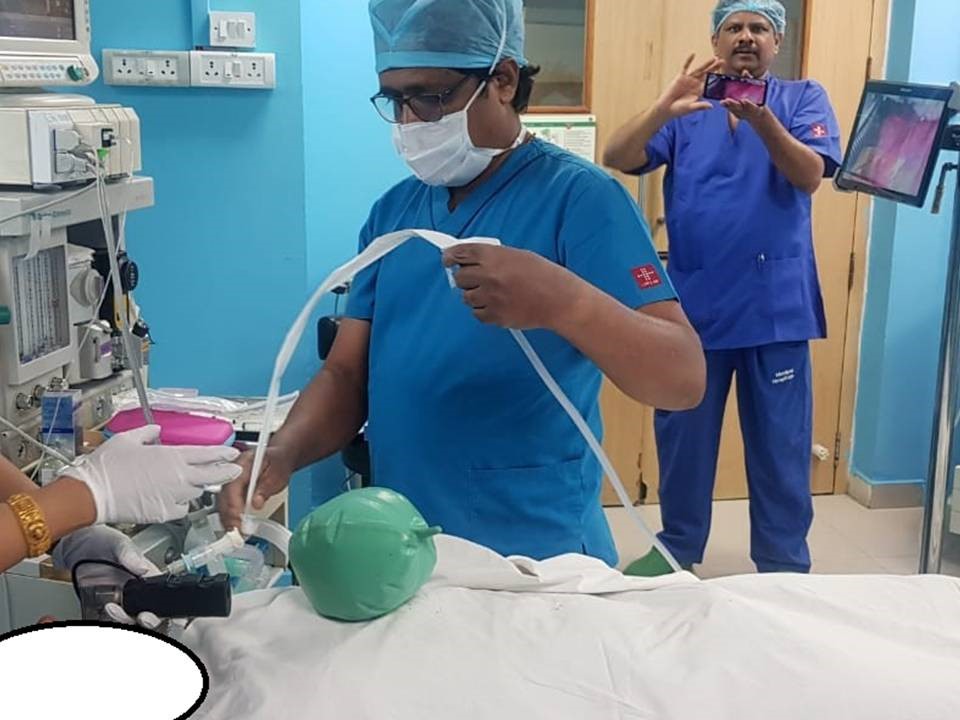

- Video Laryngoscopes: By innovating only the visualization method while using existing blade designs, we developed a Class 2 device instead of a Class 4. This approach bypassed extensive trials, saving both time and money, and translating into early profitability.
- Wireless Remote Monitoring: Addressing the need for efficient remote patient monitoring, we created a wireless version that tackles usability challenges and adheres to regulatory standards effectively. This was based on critical feedback received during PoC testing.
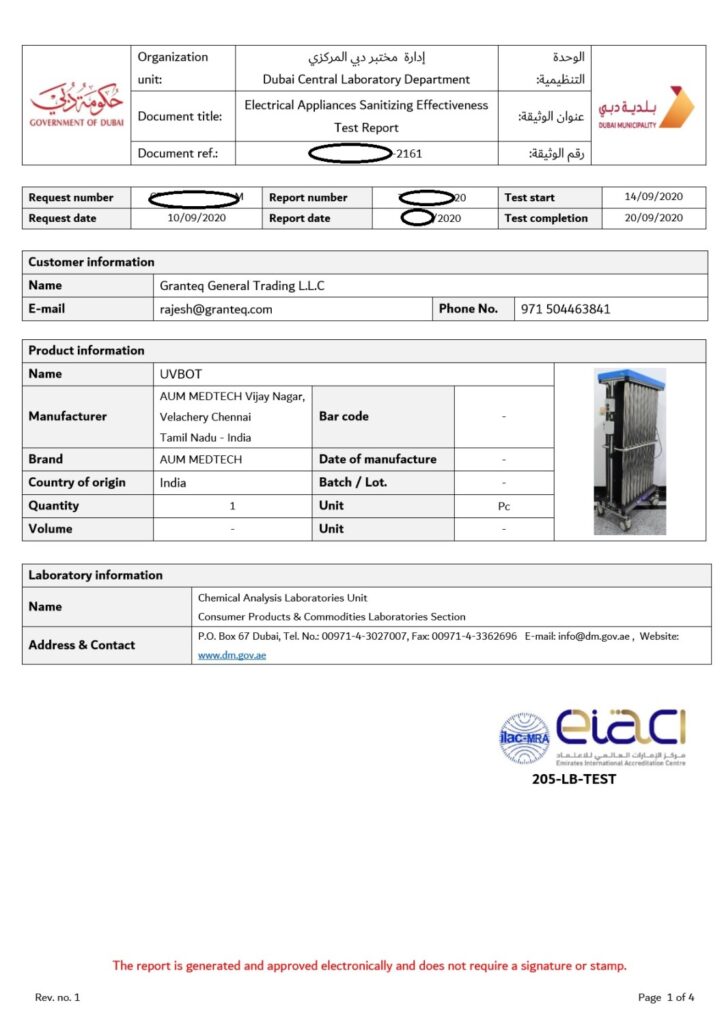
- UVC Devices: Leveraging accredited components and high-end Philips bulbs, we developed cost-effective UVC disinfection devices that meet US regulatory standards. These devices entered domestic markets within three months and expanded internationally within six months, thanks to our strategic regulatory and market expertise.
- Additionally, an Intellectual Property Rights (IPR) filing was completed before the PoC, enhancing our position in international markets by securing competitive advantage and facilitating smoother market entry.
By integrating deep medical domain knowledge with regulatory expertise and strategic planning, I deliver solutions that are not only innovative but also scalable and profitable. This experience provides valuable insights for start-ups and investors, guiding them through the complexities of market entry and ensuring successful outcomes.
